Ionic Mobility of Ions Explained
The ionic mobility of ions is a fundamental concept in physics and chemistry, describing the rate at which ions move through a medium, such as a gas or a liquid, under the influence of an electric field. This phenomenon plays a crucial role in various natural and technological processes, including atmospheric chemistry, electrochemistry, and the functioning of living organisms. In this article, we will delve into the concept of ionic mobility, exploring its underlying principles, factors influencing it, and its significance in different fields.
Key Points
- Ionic mobility is the rate at which ions move through a medium under the influence of an electric field.
- The mobility of ions is influenced by factors such as the charge and size of the ion, the viscosity of the medium, and the temperature.
- Ionic mobility plays a crucial role in atmospheric chemistry, electrochemistry, and the functioning of living organisms.
- The measurement of ionic mobility is essential in various fields, including environmental monitoring and medical research.
- Understanding ionic mobility is vital for the development of new technologies, such as fuel cells and electrochemical sensors.
Principles of Ionic Mobility

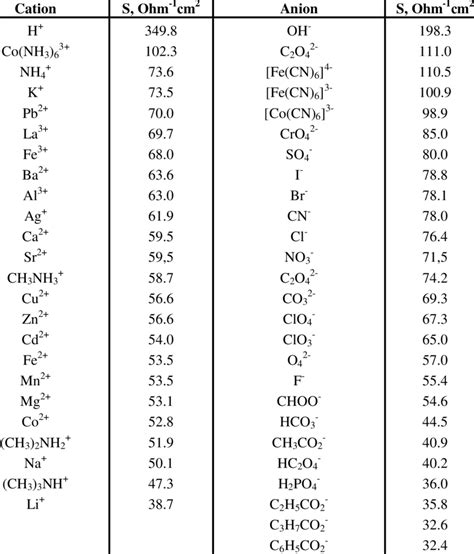
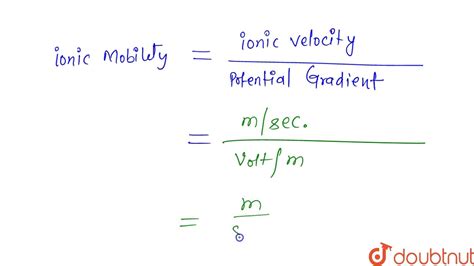
The ionic mobility of ions is determined by the balance between the electric force acting on the ion and the frictional force opposing its motion. The electric force is proportional to the charge of the ion and the strength of the electric field, while the frictional force depends on the viscosity of the medium and the size and shape of the ion. According to the Einstein-Smoluchowski equation, the mobility of an ion is given by the ratio of its charge to the frictional coefficient, which is a measure of the ion’s interaction with the surrounding medium.
Factors Influencing Ionic Mobility
The mobility of ions is influenced by several factors, including the charge and size of the ion, the viscosity of the medium, and the temperature. The charge of the ion determines the magnitude of the electric force acting on it, while the size and shape of the ion affect the frictional force opposing its motion. The viscosity of the medium, which is a measure of its resistance to flow, also plays a crucial role in determining the mobility of ions. Additionally, the temperature of the medium can influence the mobility of ions by affecting the viscosity and the kinetic energy of the ions.
| Factor | Influence on Ionic Mobility |
|---|---|
| Charge of the ion | Determines the magnitude of the electric force |
| Size and shape of the ion | Affects the frictional force opposing its motion |
| Viscosity of the medium | Opposes the motion of the ion |
| Temperature | Affects the viscosity and kinetic energy of the ions |
Significance of Ionic Mobility
The ionic mobility of ions has significant implications in various fields, including atmospheric chemistry, electrochemistry, and the functioning of living organisms. In atmospheric chemistry, the mobility of ions influences the formation and growth of aerosols, which play a crucial role in determining the Earth’s climate. In electrochemistry, the mobility of ions is essential for the functioning of devices such as fuel cells and electrochemical sensors. Additionally, the mobility of ions plays a vital role in the functioning of living organisms, where it influences the transport of ions across cell membranes and the functioning of nerve and muscle cells.
Measurement of Ionic Mobility
The measurement of ionic mobility is essential in various fields, including environmental monitoring and medical research. Several techniques are available for measuring ionic mobility, including electrophoresis, ion chromatography, and mass spectrometry. These techniques allow researchers to determine the mobility of ions in different media and under various conditions, providing valuable insights into the behavior of ions in different systems.
What is the significance of ionic mobility in atmospheric chemistry?
+The mobility of ions influences the formation and growth of aerosols, which play a crucial role in determining the Earth's climate.
How is ionic mobility measured?
+Ionic mobility is measured using techniques such as electrophoresis, ion chromatography, and mass spectrometry.
What is the role of ionic mobility in the functioning of living organisms?
+The mobility of ions influences the transport of ions across cell membranes and the functioning of nerve and muscle cells.
In conclusion, the ionic mobility of ions is a fundamental concept that plays a crucial role in various natural and technological processes. Understanding the principles and factors influencing ionic mobility is essential for predicting and controlling the behavior of ions in different systems. The measurement of ionic mobility is also vital in various fields, including environmental monitoring and medical research. By exploring the significance of ionic mobility, we can gain valuable insights into the behavior of ions and develop new technologies and applications that rely on the manipulation of ions.



